Abstract:
High-dose therapy and autologous hematopoietic cell transplantation (HDT-AHCT) is a standard of care (SOC) and potentially curative consolidation therapy for eligible patients with rel/ref lymphoma. While effective, HDT-AHCT is associated with severe regimen-related toxicities (SRRT) that affect quality of life and may increase mortality risk. SRRTs occur because of off-target cytotoxicity and vascular endothelial damage caused by HDT. Higher rates of SRRT occur in older patients because of age-related endothelial dysfunction, thus potentially limiting candidacy for AHCT. Moreover, there are limited treatment strategies to prevent SRRT. AB-205 is an engineered cell therapy consisting of allogeneic, human umbilical vein endothelial cells transduced with E4ORF1 (E-CEL® cells). AB-205’s mechanism of action is via induced expression of reparative angiocrine factors thought to accelerate tissue repair through the organ vascular stem cell niches. The proposed clinical indication of AB-205 is to prevent/reduce SRRT related to HDT.
In a previously presented Phase 1/2 study [NCT03925935], AB-205 was well tolerated, resulting in a 9% incidence of oral/GI SRRT [defined as Grade ≥3 oral mucositis, nausea, vomiting and/or diarrhea] in 35 lymphoma subjects who received BEAM (or BEAM-like) conditioned-AHCT. The incidence of oral/GI SRRT in AB-205 subjects compared favorably to an incidence of 41% seen in a contemporary retrospective observational study conducted concurrently at 2 sites during the phase 1/2 study. The median time to neutrophil engraftment was accelerated by 2 days (10 vs. 12) and length of hospital stay was shortened by 3 days (12 vs.15), respectively. In those ≥40 years-old, the oral/GI SRRT rate was 46% in the retrospective data versus 0% in subjects treated with AB-205 (unpublished; data in preparation).
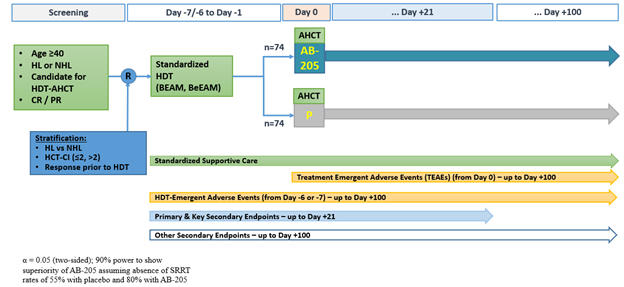
Accordingly, a phase 3, randomized (1:1), double-blind, placebo-controlled study (E-CELERATE) was initiated to further investigate the efficacy and safety of AB-205 or placebo + SOC supportive prophylaxis in AHCT-eligible subjects with lymphoma (Figure 1). Key inclusion: age ≥40; diagnosis of lymphoma and a candidate for HDT-AHCT; ECOG ≤ 2; adequate organ function. Key exclusion: prior HCT; diagnosis of CNS lymphoma; other active malignancy; history of HIV.
All subjects receive BEAM (or BeEAM) conditioning and SOC supportive prophylaxis. AB-205 IV (20×106 cells/kg) or placebo IV (diluent only) is administered on day 0 after stem cell infusion. The primary endpoint is complete response defined as the absence of oral/GI SRRT (defined above) from the time of HDT through day +21. Secondary endpoints include the duration of oral/GI SRRT; patient-reported symptom burden; duration of febrile neutropenia; time to engraftment. Approximately 140 adult patients will be enrolled across 30 sites in the U.S. Active recruitment and enrollment are on-going [NTC05181540].
Figure 1: AB-205-301 [NCT05181540] Study Schema
Here we report the study design of the AB-205 Phase III, randomized, double-blind, placebo controlled multi-center study (E-CELERATE) that has been initiated.
Tandem Meetings – Transplantation & Cellular Therapy Meetings of ASTCT and CIBMTR – February 16, 2023
Michael Scordo, MD1,2, Geoffrey Shouse, PhD, DO3, Luke Mountjoy, DO4, Jordan Gauthier, MD5,6, Bhagirathbhai Dholaria, MBBS7, Scott D. Rowley, MD, FACP8, Jeremy Pantin, MD, FACP9, Zachariah Defilipp, MD10, Monica Mead, MD11, Mehrdad Abedi, MD12, Rashmi Khanal, MD13, Umar Farooq, MD14, Joseph Bubalo, PharmD, BCPS, BCOP15, Carolyn Mulroney, MD16, Attaphol Pawarode, MD17, Muzaffar Qazilbash, MD, PhD18, Amer Beitinjaneh, MD19, Nancy Hardy, MD, FACP20, Farhad Khimani, MD21, Tsiporah Shore, MD2, Daniel O’Leary, MD22, Evandro Bezerra, MD23, Jon Arnason, MD24, Chukwuemeka Uzoka, MD25, Jason Romancik, MD26, and Paul Finnegan, MD27
1Memorial Sloan Kettering Cancer Center, New York, NY; 2Weill Cornell Medical College, New York, NY; 3City of Hope Comprehensive Cancer Center, Duarte, CA; 4Blood Cancer Institute, Denver, CO; 5Fred Hutchinson Cancer Research Center, Seattle, WA; 6University of Washington, Seattle, WA; 7Vanderbilt University Medical Center, Nashville, TN; 8Hackensack Meridian Health, Hackensack, NJ; 9TriStar Centennial Medical Center, Nashville, TN; 10Massachusetts General Hospital, Boston, MA; 11UCLA Medical Center, Los Angeles, CA; 12UC Davis Comprehensive Cancer Center, Davis, CA; 13Fox Chase Cancer Center, Philadelphia, Pennsylvania; 14University of Iowa Hospitals & Clinics, Iowa City, Iowa; 15Oregon Health and Science University Hospital, Portland, OR; 16UC San Diego Health, San Diego, CA; 17University of Michigan Comprehensive Cancer Center, Ann Arbor, MI; 18MD Anderson Cancer Center, Houston, TX; 19University of Miami Health System, Miami, FL;20University of Maryland School of Medicine, Baltimore, MD; 21Moffitt Cancer Center, Tampa, FL; 22University of Minnesota, Minneapolis, MN; 23Ohio State University, Columbus, OH; 24Beth Israel Deaconess Medical Center, Boston, MA; 25University of Illinois Cancer Center, Chicago, IL; 26Emory University, Atlanta, GA; and 27Angiocrine Bioscience, San Diego, CA
Go to Tandem Meetings – 2023